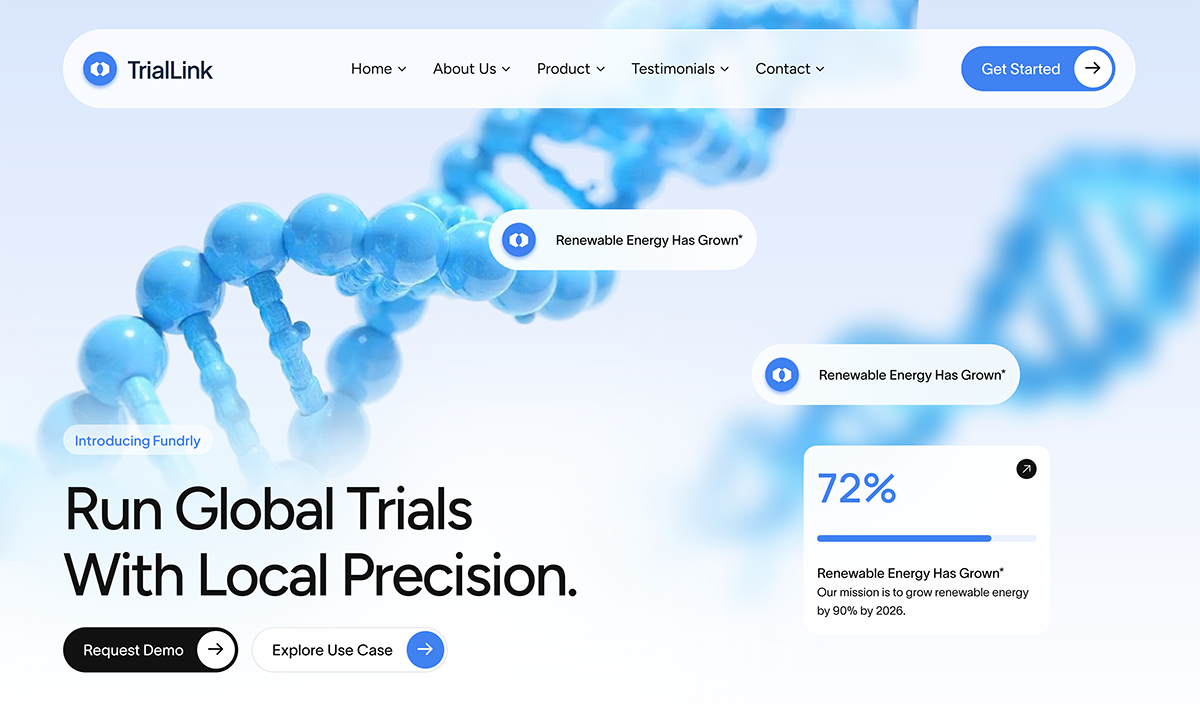
Where compliance meets clarity, speed, and scale
TriaLink Health set out to modernize global trial operations across 40+ sites and multiple continents. Their legacy systems, spreadsheets, static eCRFs, and email chains were slowing down enrollment, fragmenting data, and complicating compliance.
They needed more than a secure system. They needed a scalable, real-time collaboration portal built for decentralized studies, complex protocols, and cross-regional oversight.
Scalability partnered with TriaLink to design and launch a purpose-built digital platform that unifies operations, improves visibility, and accelerates trials — from site to sponsor.
What we did
Product Strategy
UX Design
Secure Web Development
Data Architecture
Compliance-Driven Feature Design
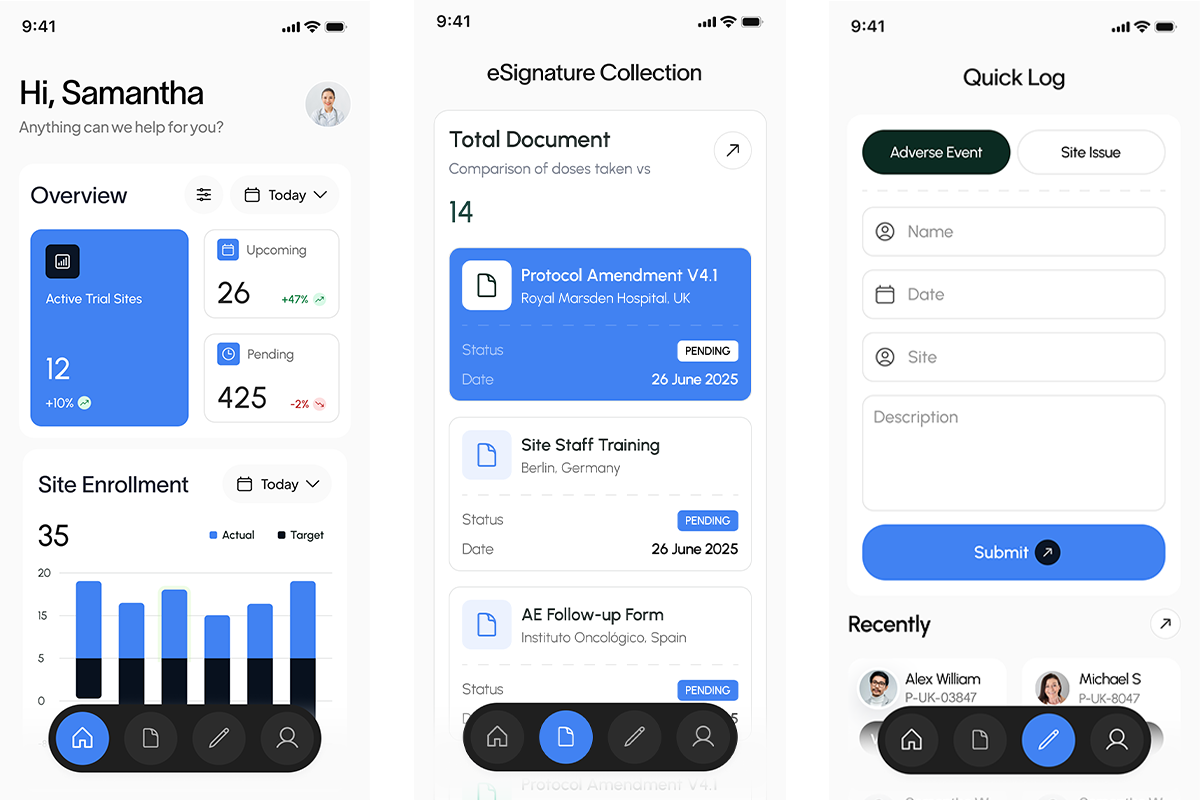
The Challenge
Legacy tools weren’t built for today’s decentralized clinical trials.
TriaLink’s trial managers and regional teams were juggling thousands of participants across dozens of sites — using siloed spreadsheets, inconsistent eCRFs, and manual tracking methods. Critical updates were delayed, compliance risks were increasing, and global visibility was nearly impossible.
Their teams needed a centralized, secure platform that could:
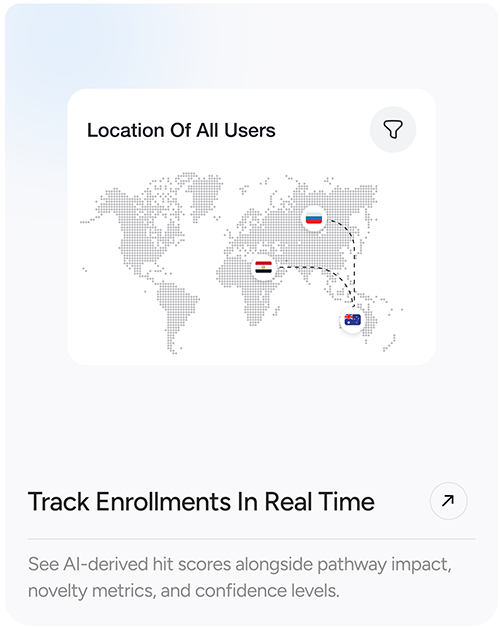
- Track enrollments in real time, across countries and protocols
- Standardize workflows across site monitors and study leads
- Ensure audit-ready documentation for regulators and sponsors
- Support rapid communication across time zones and teams
The existing patchwork of tools couldn’t keep pace. TriaLink needed a digital command center to support the future of global clinical research.
The Solution
Scalability partnered with TriaLink to design and deliver a centralized clinical trial portal -tailored to the needs of sponsors, site managers, and clinical research associates.
The result was a fully integrated, cloud-based platform built on a secure, multi-tenant architecture. Each study operates independently with shared infrastructure for compliance, reporting, and data standards.
Key features included:
- Site-level access controls with HIPAA and GDPR compliance
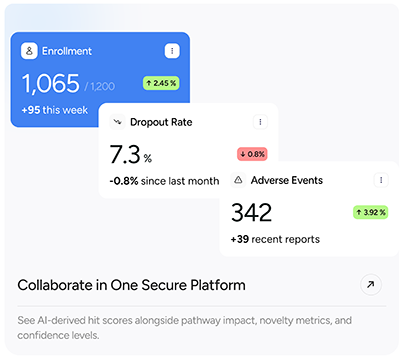
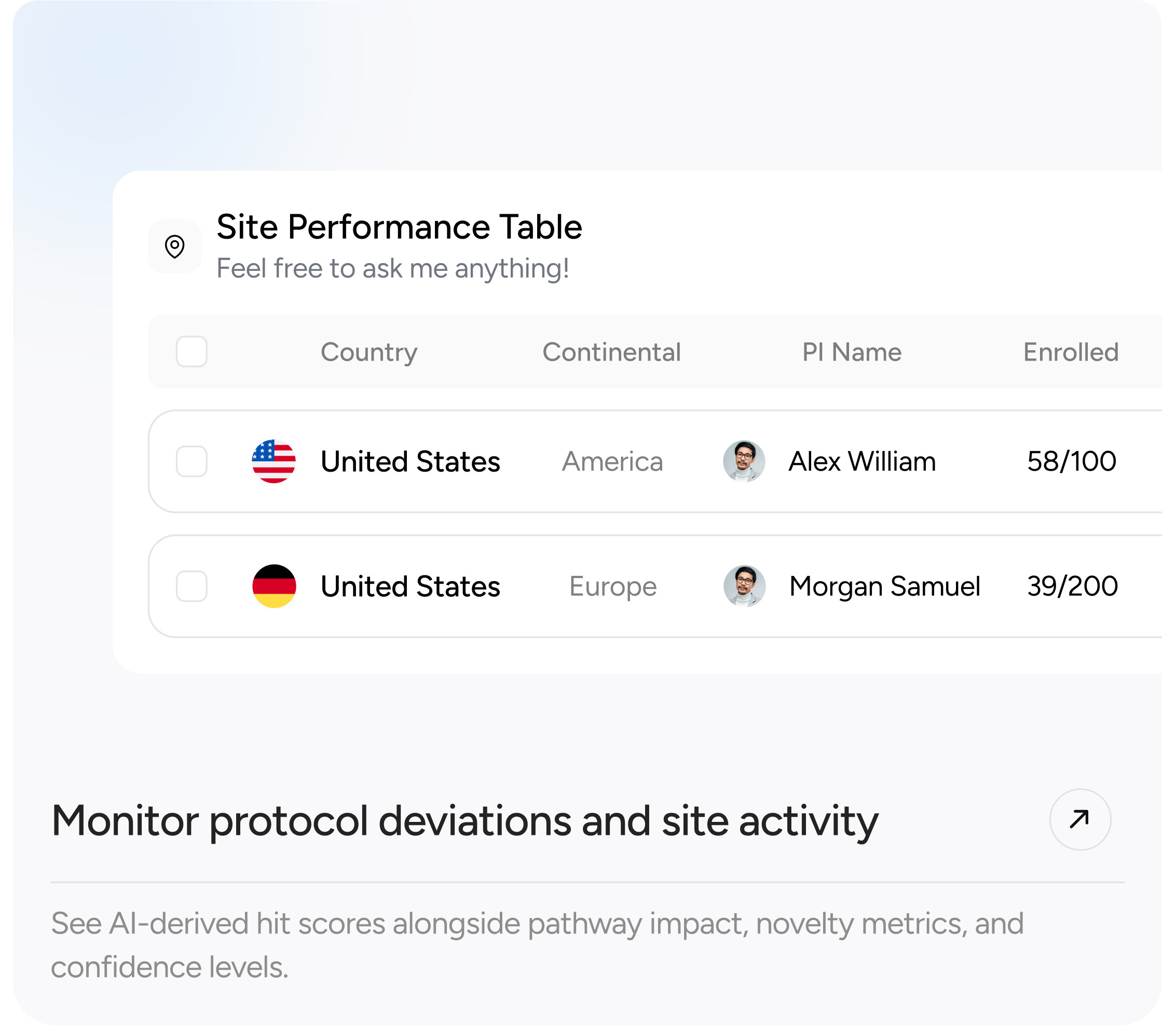
- Real-time enrollment tracking and site performance dashboards
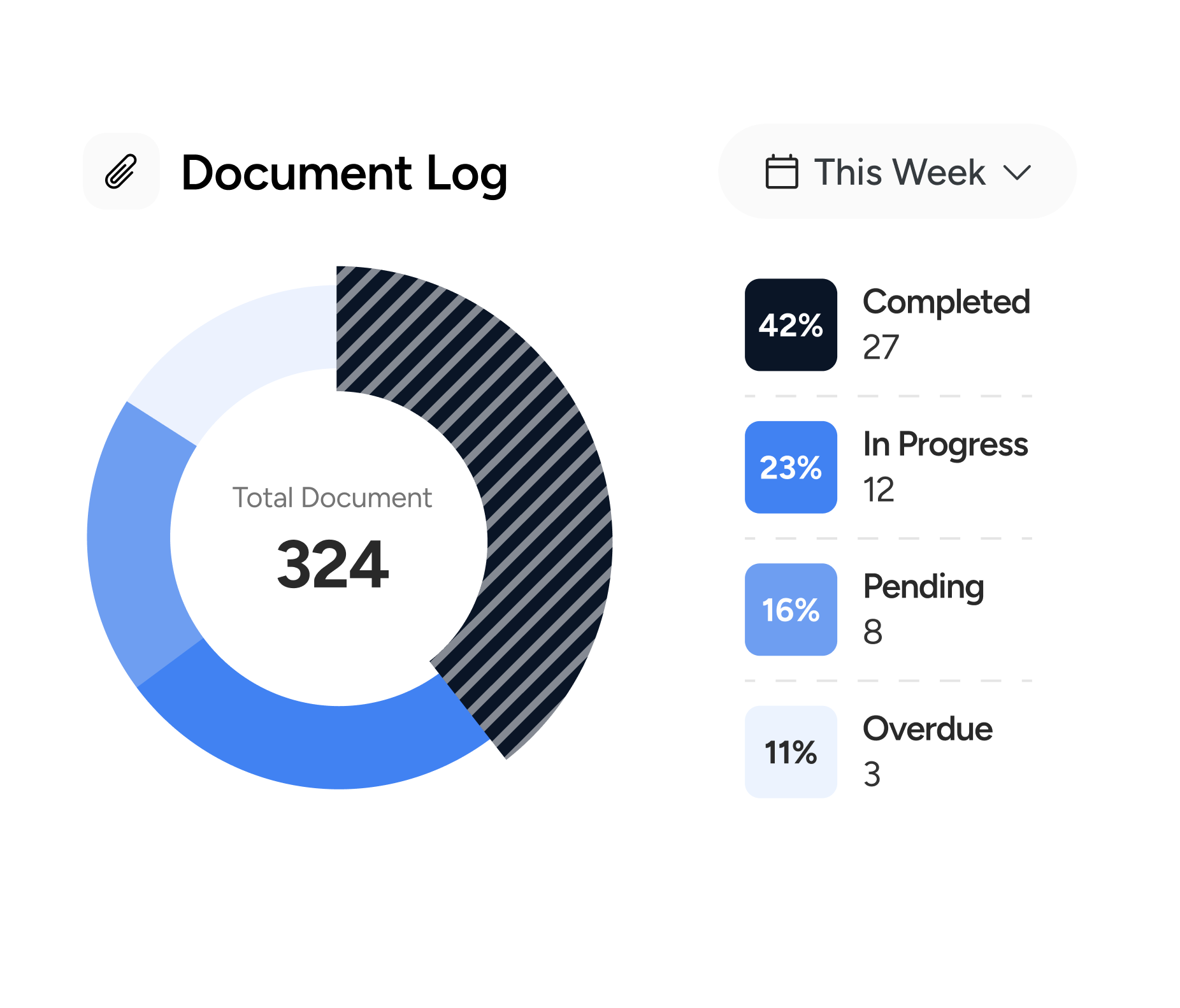
- Protocol versioning, document workflows, and audit trails
- Global trial map and KPIs by site, status, and region
- Event reporting and automated notifications for AEs, deviations, and form deadlines
Built for flexibility, the platform now supports a wide range of therapeutic areas and operational models.
The Impact
From scattered spreadsheets to one source of truth.
Since launch, the portal has transformed how TriaLink runs trials across borders. Real-time visibility has reduced enrollment lag and improved protocol adherence, while centralized document tracking has made audit prep faster and more reliable.
Sponsors and site teams now collaborate in a single, secure environment - improving transparency, accelerating reporting, and boosting operational confidence at every level.
The platform has already supported 6 therapeutic areas across 12 countries, helping TriaLink secure new global contracts and establish a digital leadership position in the CRO space.
Let's collaborate to turn your vision into reality.